Abstract: Persistent postconcussion syndrome (PPCS) after mild traumatic brain injury (mTBI) is a significant public health and military problem for which there is limited treatment evidence. The aim of this study was to determine whether forty 150 kPa hyperbaric...
Local doctor continues to push VA to approve Hyperbaric Oxygen Therapy for veterans
The AC133 antigen is a novel antigen selectively expressed on a subset of CD34+ cells in human fetal liver, bone marrow, and blood as demonstrated by flow cytometric analyses. In this study, we have further assessed the expression of AC133 on CD34+ cells in hemopoietic samples and found that there was a highly significant difference between normal bone marrow and cord blood versus aphereses (p <0.0001) but not between bone marrow and cord blood. Most of the clonogenic cells (67%) were contained in the CD34+AC133+ fraction. Compared with cultures of the CD34+AC133- cells, generation of progenitor cells in long-term culture on bone marrow stroma was consistently 10- to 100-fold higher in cultures initiated with CD34+AC133+ cells and was maintained for the 8-10 weeks of culture.
Treatment of Traumatic Brain Injury With Hyperbaric Oxygen Therapy
Hyperbaric oxygen therapy (HBOT) is defined as the use of oxygen at higher than atmospheric pressure for the treatment of underlying disease processes and the diseases they produce. Modern HBOT in which 100% O2 is breathed in a pressurized chamber dates back to the 1930s, when it was first used for treatment of decompression illness in divers. There are currently 13 FDA-approved uses for HBOT, including decompression illness, gas gangrene, air embolism, osteomyelitis, radiation necrosis, and the most recent addition—diabetic ulcers. HBOT can dramatically and permanently improve symptoms of chronic TBI months or even many years after the original head injury. This assertion is generally met with skepticism within the medical establishment because we have been taught for generations that any post-concussion symptoms persisting more than 6 months or so after a head injury are due to permanent brain damage that cannot be repaired.
The National Brain Injury Rescue and Rehabilitation Study – a multicenter observational study of hyperbaric oxygen for mild traumatic brain injury with post-concussive symptoms
The National Brain Injury Rescue and Rehabilitation Project was established as a preliminary study to test the safety and practicality of multi-center hyperbaric oxygen administration for the post-concussive symptoms of chronic mild traumatic brain injury as a precursor to a pivotal, independent, multi-center, controlled clinical trial. This report presents the results for 32 subjects who completed a preliminary trial of hyperbaric oxygen several years before the passage of the 21 st Century Cures Act. This study anticipated the Act and its reassessment of clinical research. Subjects received 40-82 one-hour treatments at 1.5 atmospheres absolute 100% oxygen. Outcome measures included repeated self-assessment measures and automated neurocognitive tests. The subjects demonstrated improvement in 21 of 25 neurocognitive test measures observed. The objective neurocognitive test components showed improvement in 13 of 17 measures. Earlier administration of hyperbaric oxygen post injury, younger age at the time of injury and hyperbaric oxygen administration, military status, and increased number of hyperbaric oxygen administrations were characteristics associated with improved outcomes. There were no adverse events. Hyperbaric oxygen was found to be safe, inexpensive and worthy of clinical application in the 21 st Century model of facile data collection provided by recent research regulatory shifts in medicine. The study was approved by the ethics review committee of the Western Institutional Review Board (WIRB; Protocol #20090761).
Effect of hyperbaric oxygen therapy on chronic neurocognitive deficits of post-traumatic brain injury patients: retrospective analysis.
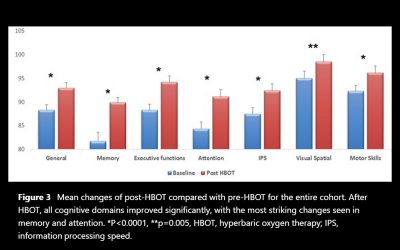
The aim of the study is to evaluate the effect of hyperbaric oxygen therapy (HBOT) in participants suffering from chronic neurological deficits due to traumatic brain injury (TBI) of all severities in the largest cohort evaluated so far with objective cognitive function tests and metabolic brain imaging. A retrospective analysis was conducted of 154 patients suffering from chronic neurocognitive damage due to TBI, who had undergone computerised cognitive evaluations pre-HBOT and post-HBOT treatment. The average age was 42.7-14.6 years, and 58.4% were men. All patients had documented TBI 0.3-33 years (mean 4.6-5.8, median 2.75 years) prior to HBOT. HBOT was associated with significant improvement in all of the cognitive domains, with a mean change in global cognitive scores of 4.6-8.5 (p<0.00001).
Evidence brief: hyperbaric oxygen therapy (HBOT) for traumatic brain injury and/or post-traumatic stress disorder.
This report is a product of the VA Evidence-based Synthesis Program. The purpose is to provide “timely and accurate syntheses of targeted healthcare topics. to improve the health and healthcare of Veterans”. The authors have made a comprehensive search and analysis of the literature and make recommendations to assist clinicians in dealing with veterans suffering from either traumatic brain injury (TBI) or post-traumatic stress disorder (PTSD). The report is timely and of great potential impact given the vigorous and lengthy debate among hyperbaric physicians and lay people determined to find an answer for the large numbers of veterans deeply affected with some combination of PTSD and post-concussion dysfunction.
HYPERBARIC OXYGEN THERAPY- BASICS AND NEW APPLICATIONS
Hyperbaric oxygen therapy (HBOT) serves as primary or adjunctive therapy for a diverse range of medical conditions. The indication for HBOT can be related to either pressure (decompression sickness or air emboli) or tissue hypoxia. It is now realized, that the combined action of hyperoxia and hyperbaric pressure, leads to significant improvement in tissue oxygenation while targeting both oxygen and pressure sensitive genes, resulting in improved mitochondrial metabolism with anti-apoptotic and anti-inflammatory effects. Clinical studies published in recent year’s present convincing evidence that HBOT can be the coveted neurotherapeutic method for brain repair. Here we discuss the multi-faceted role of HBOT in wound care in general and in neurotherapeutics in detail.
Beneficial Effect of β-Elemene Alone and in Combination with Hyperbaric Oxygen in Traumatic Brain Injury by Inflammatory Pathway.
Present study evaluates the neuroprotective effect of β-elemene alone and in combination with hyperbaric oxygen (HO) in traumatic brain injury (TBI). TBI was induced by dropping a weight from a specific height. All the animals were separated in to five groups (n=20) like control group; TBI group; β-elemene treated group which receives β-elemene (100 mg/kg, i.p.) half an hour after the injury; HO group which receives hyperbaric oxygen therapy and β-elemene + HO group which receives β-elemene (100 mg/kg, i.p.) half an hour after the injury and hyperbaric oxygen therapy. Neurological function was assessed to evaluate the effect of β-elemene in TBI rats. Thereafter level of inflammatory cytokines and expression of protein of inflammatory pathway was assessed in the brain tissues of TBI rats.
The protection effect and mechanism of hyperbaric oxygen therapy in rat brain with traumatic injury.
To investigate the effect of hyperbaric oxygen therapy (HBOT) on traumatic brain injury (TBI) outcome. The modified Marmarou’s weight drop device was used to generate non-lethal moderate TBI rat model, and further developed in vitro astrocytes culturing system. Then, we analyzed the expression changes of interested genes and protein by quantitative PCR and western blot. Multiple HBO treatments significantly reduced the expression of apoptosis promoting genes, such as c-fos, c-jun, Bax and weakened the activation of Caspase-3 in model rats. On the contrary, HBOT alleviated the decrease of anti-apoptosis gene Bcl-2 and promoted the expression of neurotrophic factors (NTFs), such as NGF, BDNF, GDNF and NT-3 in vivo. As a consequent, the neuropathogenesis was remarkably relied with HBOT.
Case control study: hyperbaric oxygen treatment of mild traumatic brain injury persistent post-concussion syndrome and post-traumatic stress disorder.
Mild traumatic brain injury (TBI) persistent post-concussion syndrome (PPCS) and post-traumatic stress disorder (PTSD) are epidemic in United States Iraq and Afghanistan War veterans. Treatment of the combined diagnoses is limited. The aim of this study is to assess safety, feasibility, and effectiveness of hyperbaric oxygen treatments (HBOT) for mild TBI PPCS and PTSD. Thirty military subjects aged 18-65 with PPCS with or without PTSD and from one or more blast-induced mild-moderate traumatic brain injuries that were a minimum of 1 year old and occurred after 9/11/2001 were studied. The measures included symptom lists, physical exam, neuropsychological and psychological testing on 29 subjects (1 dropout) and SPECT brain imaging pre and post HBOT.