The Placebo Problem in HBOT Research
Hyperbaric oxygen therapy (HBOT) has long shown promise in treating a range of neurological conditions, from traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) to post-COVID symptoms. Yet, clinical research on HBOT faces a unique obstacle: the difficulty of creating a true placebo control. In randomized controlled trials, the gold standard of medical research, participants ideally should not know whether they are receiving the actual treatment or a placebo. This minimizes bias and ensures that improvements can be attributed to the treatment itself rather than patient expectation.
But HBOT complicates this process. Patients can often feel physical sensations associated with increased pressure inside the hyperbaric chamber—ear popping, chest tightness, and even changes in balance—making it nearly impossible to design a placebo that truly mimics the treatment. As a result, many HBOT studies have been criticized for potential bias, leaving researchers and clinicians searching for more reliable evidence.

A Creative Solution: The Sham Group
A new study published in Scientific Reports by Weaver et al. (2025), however, has found a way to overcome this barrier. Using a carefully designed sham protocol, the researchers were able to conduct a double-blind, randomized, sham-controlled clinical trial on HBOT for persistent brain injury symptoms—a rare and important achievement in the field.
The sham group was designed to mimic the HBOT experience as closely as possible without delivering therapeutic oxygen. Participants in the sham group entered the chamber, heard the machinery, and even felt a slight increase in pressure that required them to equalize their ears—sensations very similar to actual HBOT. To simulate this, the chamber was pressurized to only 0.007 ATA, equivalent to approximately 2.8 inches underwater. This subtle pressurization provided the physical sensation of being in a hyperbaric environment but did not confer the physiological benefits of true hyperbaric exposure, which in the treatment group reached 1.5 ATA, equivalent to being 16.5 feet underwater. Importantly, the sham participants still breathed only room air.
By creating this realistic “fake” treatment environment, the researchers successfully kept participants unaware of whether they were in the treatment or control group. This allowed them to test HBOT against a genuine placebo condition, ensuring that improvements could be attributed to the therapy itself rather than to expectation or the experience of being in a chamber.
With this design in place, the researchers were able to accurately measure the true impact of HBOT on brain injury symptoms—leading to some groundbreaking findings.
Key Findings from the Study
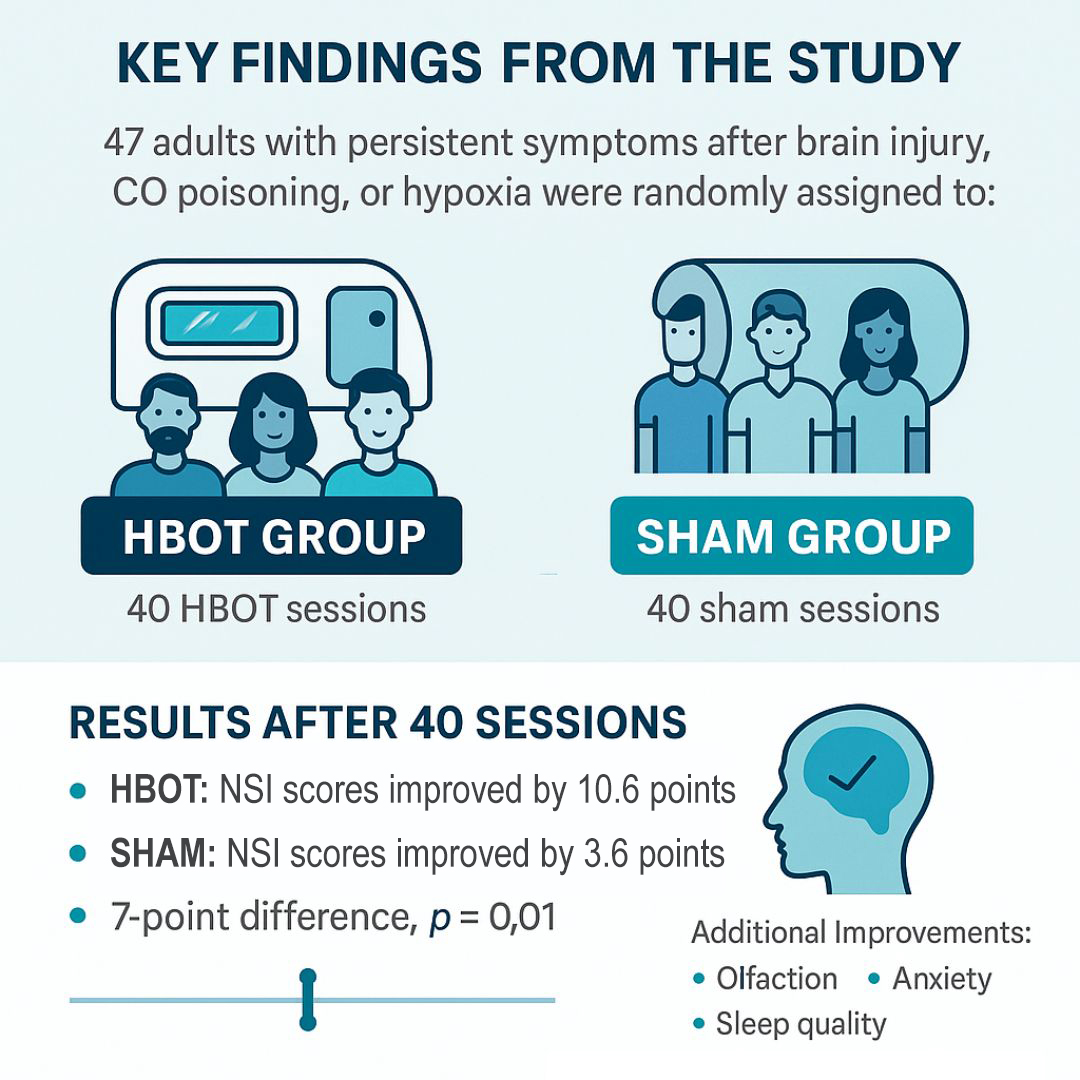
The trial enrolled 47 adults who had persistent symptoms at least six months after a brain injury, including TBI, carbon monoxide poisoning, or hypoxia. Participants were randomly assigned to either receive 40 HBOT sessions at 1.5 ATA or 40 sham sessions with only minimal pressurization and room air. Importantly, the sham protocol was designed to feel real enough to maintain blinding—both groups had to equalize their ears, for example—making it one of the most rigorously controlled HBOT studies to date.
Results after 40 Sessions (13 Weeks):
- HBOT group improved their Neurobehavioral Symptom Inventory (NSI) scores by 10.6 points (p < 0.001).
- Sham group improved by 3.6 points (p = 0.02).
- The between-group difference of 7 points was statistically significant (p = 0.01).
In addition to general symptom relief, the HBOT group showed specific improvements in olfaction (sense of smell), anxiety, sleep quality, and vestibular complaints (balance/dizziness). Both groups improved in depression, headaches, and PTSD symptoms—likely reflecting a combination of placebo effects and the structured environment of the trial.
Additional Treatments:
Three months later, all participants were offered 40 open-label HBOT sessions. Those who had already received HBOT and continued for a total of 80 sessions showed even greater improvements, with sustained benefits at 12 months and beyond. Importantly, effects were most durable in the HBOT group, while improvements in the sham group faded over time.
A subgroup analysis also revealed that participants with PTSD symptoms benefited the most, showing significant reductions in neurobehavioral symptoms compared to those without PTSD.
Long-Term Benefits and Sustainability of HBOT
An important aspect of this trial was its extended follow-up, which included assessments at 24 and 36 months after randomization. Notably, this is the only known HBOT study for brain injury that incorporated outcomes at 36 months. Of the 13 participants eligible for this long-term analysis, results demonstrated a reduction in self-reported post-concussion symptoms.
These findings are significant because they highlight that the benefits of HBOT are not only immediate but also sustainable over time. For individuals living with the persistent effects of TBI and PTSD, long-term improvement means more than temporary relief—it suggests that HBOT may help support lasting recovery and quality of life well beyond the initial course of treatment.
A Turning Point for HBOT and Research
This study represents one of the clearest and most rigorous demonstrations to date that Hyperbaric Oxygen Therapy (HBOT) delivers real, measurable benefits for people living with persistent symptoms after traumatic brain injury (TBI) and PTSD. Patients who received HBOT reported greater improvements than those in the sham group, and these benefits were sustained over time—especially with extended treatment up to 80 sessions. The findings confirm what many in the HBOT community have long observed: HBOT is not simply a placebo, but a therapy with genuine healing potential.
A major breakthrough of this trial lies in its research methodology. By successfully designing a sham protocol that mimicked the chamber experience while maintaining blinding, researchers addressed one of the biggest challenges in HBOT studies: ruling out placebo effects. This innovation strengthens the evidence base for HBOT and provides a blueprint for future trials to produce more reliable, clinically meaningful results.
For patients, these findings are particularly encouraging. Persistent symptoms such as headaches, sleep disturbances, dizziness, anxiety, and cognitive difficulties often linger for years after injury. The study demonstrates that HBOT can offer durable relief even long after the initial trauma, with extended treatment yielding greater improvements than the standard 40-session course.
On a broader scale, this trial may shift the conversation within mainstream medicine. By directly countering criticism that HBOT’s benefits are placebo-driven, it establishes both the legitimacy of HBOT as a treatment option for TBI and PTSD and a rigorous framework for advancing its integration into clinical practice.
References
- Weaver, Lindell K., et al. “A double-blind randomized trial of hyperbaric oxygen for persistent symptoms after brain injury.” Scientific Reports, vol. 15, no. 1, 26 Feb. 2025, https://doi.org/10.1038/s41598-025-86631-6.
